Cleaning equipment used to clean and thus ensure the safety of workers includes all measures which enable the removal of solid and liquid dirt which can occur at the workplace.
For household work and food industry, the use of high quality cloths and wipers is recommended.
For professional removal of chemical-based stains we recommend the use of sorbents and emergency kits.
Sorbents
A sorbent is a fragmented material which due its fragmentation has large surface area.
The characteristic feature of sorbents is their absorbing capacity of 100% to even 400%.
Types of sorbents according to their structure and used material
Based on the physical properties there are powder sorbents - made of fragmented solid body of a compact structure, and textile sorbents - made in the form of mats, cushions, sleeves and others.
Mineral sorbents
They rank among powder sorbents and are available as small pellets which thanks to their miniature size can reach even the tightest gaps and absorb dangerous liquids from any type of surface.
They are made of a rock material subject to physical and thermal treatment whose purpose is to extend the absorption surface.
Sorbents which consist of pellets with a small diameter (0.3-1mm) are suitable for use inside buildings - outside they may be blown away by the wind or otherwise affected by the weather.
Sorbents which consist of pellets with a medium and large diameter (1-3mm) are heavier and resistant to the wind. They are suitable for use both inside buildings and in the open air.
Natural sorbents
They also rank among powder sorbents. They are made of bark which after fragmentation and special treatment gains absorptive properties and becomes more durable.
Textile sorbents
Textile sorbents are manufactured in the form of absorptive mats. These mats are designed to be unfolded at the place of the leak of a harmful or dangerous substance where they will quickly neutralize the hazardous substance.
Textile sorbents are made of polypropylene nonwoven fabric which makes them light and enables them to float on the surface of water which makes them suitable for neutralization of risks which occur in waterbodies. Sorbents demonstrate an absorption level of 100%.
Textile sorbents are divided into groups intended for the neutralization of various hazards. Before using a given sorbent, you should verify its field of application.
Textile sorbents are an ideal solution to prevent the spread of pools of dangerous substances. They may also be used to cover smaller work areas and smaller pools. Rolls of absorptive mats are used to secure larger pools.
Absorbent sleeves
Absorbent sleeves are a type of textile sorbents and are used to collect impurities such as oil spills from the surface of the water.
Depending on the type and specific field of use, absorbent sleeves have different length, diameter and connection type.
Classification of absorbents according to neutralized substances
Oil sorbents
Hydrophobic sorbents which remove impurities through selective absorption. These sorbents do not absorb water which makes them suitable for use on the ground and on water surface.
High absorptive properties enable quick and efficient elimination of oil spills over a very short period of time regardless of their density.
Principally used for the neutralization of:
- oils,
- petroleum derivatives (gasoline, petroleum),
- any hydrocarbon.
Chemical sorbents
Hydrophilic sorbents intended for absorption of chemicals including aggressive acids and bases*. These sorbents are ideal for use in laboratories as protective equipment, in fuel and chemical storages and as a protection against leaks during transport.
They may be used inside buildings or in the open air.
They are principally used for the neutralization of chemicals (paints, varnishes, diluents) and aggressive acids and bases.
Chemical resistance table sorbentów*:
| Chemical | White Oil Only Absorbants | Yellow Chemical Absorbants |
|---|---|---|
| Acetone | • | • |
| Acrolein | • | • |
| Acrylonitrile | • | |
| Acetaldehyde | • | |
| Allyl Alcohol | • | |
| Amyl Alcohol | • | |
| Benzyl Alcohol | • | |
| Butyl Alcohol | • | • |
| Ethyl Alcohol | • | • |
| Isobutyl Alcohol | • | • |
| Isopropyl Alcohol | • | • |
| Methyl Alcohol | • | • |
| Propyl Alcohol | • | • |
| Ammonia (Anhydrous) | • | • |
| Aniline | • | |
| Sodium Nitrate | • | |
| Silver Nitrate | • | |
| Benzene | • | • |
| Ethyl Benzene | • | • |
| Benzonitrile | • | |
| Gasoline | • | • |
| Acetic Anhydride | • | |
| Bromine | • | |
| Butylamine | • | |
| Quinoline | • | |
| Acetyl Chloride | • | |
| Benzoyl Chloride | • | |
| Stannic Chloride | • | |
| Ethyl Chloride | • | • |
| Methyl Chloride | • | • |
| Sodium Chloride | • | |
| Chlorobenzene | • | |
| Chloroform | • | • |
| Clorox (Full Strength Bleach) | • | |
| Hydrogen Cyanide | • | • |
| Cyclohexane | • | • |
| Carbon Tetrachloride | • | • |
| Detergents | • | |
| Dichlorobenzene | • | • |
| Diethylamine | • | • |
| Dinitrobenezene | • | • |
| Dioxan | • | |
| Carbon Disulfide | • | |
| Acrylic Emulsions | • | |
| Ether | • | • |
| Diethyl Ether | • | • |
| Ethyl Ether | • | • |
| Methyl Ether | • | • |
| Petroleum Ether | • | • |
| Phenol | • | |
| Formaldehyde | • | |
| Disooctyl Phthalate | • | • |
| Ethylene Glycol | • | |
| Propylene Glycol | • | • |
| Triethylene Glycol | • | • |
| Hexane | • | • |
| Heptane | • | • |
| Hydrazine | • | |
| Keytones | • | • |
| Cresol | • | • |
| Xytene | • | • |
| Acrylic Acid | • | |
| Aminobenzoic Acid | • | |
| Nitric Acid | • | |
| Benzoic Acid | • | |
| Boric Acid | • | |
| Chloroacetic Acid | • | |
| Chlorosulfonic Acid | • | |
| Hydrochloric Acid | • | |
| Chromic Acid (50%) | • | |
| Citric Acid | • | |
| Hydrofluoric Acid | • | |
| Phosphoric Acid | • | |
| Isobutyric Acid | • | • |
| Isopropyl Acid | • | • |
| Carbolic Acid | • | |
| Butyric Acid | • | • |
| Butyric Acid | • | • |
| Formic Acid | • | |
| Nitrobenzoic Acid | • | |
| Acetic Acid | • | |
| Glacial Acetic Acid | • | |
| Glacial Acetic Acid | • | |
| Oleic Acid | • | • |
| Propionic Acid | • | • |
| Sulfuric Acid | • | |
| Methyl Ethyl Ketone | • | • |
| Urine | • | |
| Hydrogen Peroxide | • | |
| Kerosene | • | • |
| Naphthalene | • | • |
| Nitrobenzene | • | |
| Nitrotoluene | • | • |
| Vinegar | • | |
| Amyl Acetate | • | • |
| Butyl Acetate | • | • |
| Ethyl Acetate | • | • |
| Isopropyl Acetate | • | • |
| Vinyl Acetate | • | • |
| Octane | • | • |
| Corn Oil | • | • |
| Linseed Oil | • | • |
| Mineral Oil | • | • |
| Fuel Oil | • | • |
| Castor Oil | • | • |
| Silicone Oil | • | • |
| Motor Oil | • | • |
| Synthetic Motor Oil | • | • |
| Gearbox Oil | • | • |
| Lubricating Oil | • | • |
| Transformer Oil | • | • |
| Cottonseed Oil | • | • |
| Olive Oil | • | • |
| Aviation Fuel | • | • |
| Paraffin | • | • |
| Brake Fluid | • | • |
| Sodium Hypochlorite | • | |
| Propanol | • | |
| Ethyl Propionate | • | • |
| Methyl Propionate | • | • |
| Resorcinol | • | |
| Plating Solutions | • | |
| Soap Solution (concentrated) | • | • |
| Salt Solutions (metallic) | • | |
| Sucrose | • | |
| Starch | • | |
| Styrene | • | • |
| Tannic Acid | • | |
| Turpentine | • | • |
| Perchloroethylene | • | • |
| Toluene | • | • |
| Trichloroethylene | • | • |
| Chlorine Water | • | |
| Aqua Regia | • | |
| Ammonium Hydroxide | • | • |
| Magnesium Hydroxide | • | |
| Potassium Hydroxide | • | |
| Sodium Hydroxide | • | |
| Calcium Hydroxide | • | |
| Sodium Bicarbonate | • |
Emergency kits, additional equipment
Emergency kits usually consist of a certain number of sorbents intended for neutralization of various substances and additional equipment whose purpose is to provide protection in factories and storehouses to ensure the safety of workers and enable quick neutralization in case of danger.
Due to the possibility of carrying / transport emergency kits are suitable for use as protective equipment during transportation of chemicals, oil, petroleum and others. They enable quick reaction which minimizes potential damage and risk.
Additional equipment, such as canisters for gathering oil, barrels, bags with security features enable direct neutralization of risk agents or securing the used sorbent.
Emergency kits are available in many variations and may be adjusted to individual needs.
List of standards for cleaning equipment
BS476 (EN476)
General requirements for elements of gravitational sewage systems.
The standard contains requirements for elements of gravitational sewage systems. It also provides basic definitions.
BS7959
Materials used for the control of liquid spillages. Classification of sorbents.
Cleaning cloths
Cleaning cloths are designed (structure, area density, surface) in such a way as to remove and absorb as much dirt and impurities as possible. Cleaning cloths are an alternative to seemingly cheaper products which are frequently used for cleaning purposes, such as paper, sometimes cheap paper towels or pieces of fabric or cotton scraps. Large and tightly wound rolls of cleaning cloth - though their unit price may seem high - compensate for price with their high quality, absorbing capacity and undoubtedly higher efficiency.
Due to their properties cleaning cloths are used for:
- Drying hands after washing,
- Maintaining a workplace clean – removing water, oils, organic and food stains,
- Cleaning and disinfection performed by cleaning personnel,
- Wiping, cleaning and polishing desktops,
- Cleaning and disinfection of measuring devices,
- Maintenance of machines, removing oils from the machines,
- Maintaining beauty salons, hairdresser’s, tanning salons clean,
- Cleaning glass panes, mirrors and other glass elements,
- Removing liquid spillages,
- Cleaning with the use of household cleaning products.
Thanks to the versatility of cleaning cloths they may be used in various industries, e.g. food industry, catering, hotels, car wash facilities, printing houses, the industry (cleaning machines), laboratories, repair workshops, tanning salons, beauty and hairdressing salons, hospitals, facilities with high hygiene requirements and others.
Material
Cellulose fibers are manufactured from cellulose pulp from softwood (from coniferous trees) and hardwood (deciduous trees). This process is referred to as sulfate or sulfite delignification, i.e. removal of lignin and resin from plant tissue.
Fiber bleaching
The aim of bleaching is to clean the fibers to obtain a pulp of a lighter colour and make the fibers comply with safety requirements for hygienic products and, in some cases, requirements related to food safety.
There are various methods of pulp bleaching: ECF (Elementary Chlorine Free) which uses chlorine dioxide and TCF (Totally Chlorine Free) which uses ozone, oxygen and hydrogen peroxide.
Chemical additives
Chemical substances used in the production process, as well as functional additives, were evaluated in terms of their ecologicality, occupational health and safety rules and product safety.
Among the functional additives there are:
- wet strength additives,
- dry strength additives ,
- colorants,
- fixers,
- optical brighteners,
- glue,
- emollients.
- Operational additives include:
- stabilizers,
- protective agents,
- cylinder coating,
- defoamers,
- dispersive agents,
- surface active agents,
- pH regulators,
- retention aids,
- dehydrating agents.
Product safety
This product complies with the legal requirements concerning food safety.
Environmental Product Declaration (EPD)
EPD is a document which reports environmental data of products and their life cycle assessment. It is a comparative, reliable monitoring system updated on a continuous basis which quantifies the environmental impact of a product. It provides information on environmental impact of the product throughout its entire life cycle - from the moment of its production from raw materials to the moment of utilization.
Utilization
This product is used mainly in the production processes and after use may be contaminated with various substances. Based on these substances, the appropriate utilization method should be used. We suggest to verify local regulations in terms of utilization.
The packaging may be recycled (material or energy).
List of plastics used in the production of cleaning equipment
Polypropylene
It is a polymer which ranks among the polyolefins. It is manufactured in the process of low pressure polymerization of propylene. Products made of this material are usually marked with a PP symbol. Polypropylene is a thermoplastic hydrocarbon polymer, i.e. in high temperature it may turn into liquid and then solidify when the temperature drops, without any change to its chemical properties.
The production with the use of polypropylene pellets is conducted according to one of the following methods: injection moulding or extrusion moulding. Injection moulding is used when there is a need to make objects with thin walls, complicated shape or large surface. It is used to manufacture many household articles, such as transport boxes, containers, various packages etc. Products of injection moulding are characterized by their stiffness and fine polish.
Extrusion moulding is used in the production of: tubes, insulation of steel tubes, wire insulation, plates, various profiles, foil, fibers. Polypropylene is used for the production of plastic elements, buckets, squeezers, mop handles etc.
Benefits
High impact resistance (when compared to PVC), low specific weight 0.91g/m³, high abrasion resistance, thermal stability up to 100°C, high elasticity, electrical insulation, non-toxicity, very high chemical resistance.
Application
Polypropylene is used in:
- production of household products: buckets, dustpans, sweepers, brushes, handles, freezer containers etc.;
- packaging: cheese and yogurt cups, food articles;
- electronic and electrotechnical industries;
- automotive industry etc.
Chemical resistance table*
| Substance | Chemical resistance |
|---|---|
| Acids | • |
| Bases | • |
| Solutions of salts | • |
| Solvents | • |
| Water | • |
| Aromatic hydrocarbons | |
| Chlorinated hydrocarbons | |
| Oils | • |
| Fats | • |
| Benzene | |
| Ligroin | |
| Alcohols | • |
| Fruit juices | • |
| Milk | • |
Polyethylene
Or polyethene – ethene polymer. In the industry it is marked with a PE symbol. It is a white porous substance or white powder, specific gravity 0.92-0.97g/cm³, melting temperature 110-137°C, thermoplastic. It is obtained as a result of ethene (ethylene) polymerization. Polyethylene has dielectric properties, demonstrates high mechanical resistance and resistance to chemicals and low temperatures of up to -50°C.
Polyethylene fibers rank among chemical fibers with the highest mechanical resistance. Polyethylene is used in the production of elements which have to be more elastic and resistant to cracks: snap fasteners, buckles. Polyethylene is also used in the production of bin bags.
Benefits
Very high resistance to wear and abrasion (even in case of high speeds, abrading and sliding loads), size stability (does not absorb moisture), high mechanical resistance (even in low temperatures), resistance to aggressive agents, physiologically safe, approved for contact with foodstuffs, it reduces the continuous and impact noise.
Application
Polyethylene is used in:
- food industry,
- household industry,
- machines parts.
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| resistant - 3% swelling or 0.5% weight loss; tensile elongation at break without any significant changes | |
| not resistant - 8% swelling or 5% weight loss and/or tensile elongation at break reduced by 50% | |
| conditionally resistant - 3% swelling, 0.6 to 5 % weight loss | |
| X | prohibited use |
Poly(ethylene terephthalate) - PET regrind
It is a terephthalic acid and ethylene glycol based polyester. It is a high molecular compound (polymer) with a linear structure which is a product of polycondensation. The final product is a thermoplastic polyester suitable for many applications.
Benefits
It has dielectric properties, has high mechanical resistance and is very durable; resistance to ageing and light; resistant to high and low temperatures; resistant to diluted acids and bases, oils, fats, aliphatic and aromatic hydrocarbons, bleaching agents. It is characterized by its low moisture absorption, it is physiologically inert and approved for contact with foodstuffs (sterilized with the use of ethylene oxide or by ultraviolet light irradiation).
Application
The most important fields of application of PET are: machine industry, automotive industry, household products, electrotechnical industry, electronics. It is used in the production of coloured or black bristle for the production of brooms, sweepers and brushes.
Due to its properties PET is often used in the production of packaging for, among others, bottles, in the textile industry and construction industry. PET is used in the production of bristle for brooms, brushes, sweepers etc.
Polyurethane
Polyurethane (PUR or PU) is a polymer made by addition polymerization of multifunctional isocyanates to amines and alcohols. Polyurethane polymers are melted more easily than polyamides which makes them easier to process. However, they demonstrate lower mechanical resistance. Polyurethane is indispensable in the production of massage, bath and kitchen sponges.
Benefits
Temperature range from -30°C to 80°C, resistant to oils and gasoline, high resistance to tear and fracture.
Application
The most important field of application of polyurethane is the production of sponges. They are used in large quantities in: furniture, automotive, footwear, textile industries and in the production of sponges for cleaning purposes etc.
Materials used in the production of synthetic fibers:
- PET bristle – non-split: PET regrind; PET preform, colorant,
- PET bristle – split: PET regrind; PET preform; polypropylene; polyethylene, colorant,
- Polypropylene bristle – PP: polypropylene; colorant.
Cleaning chemicals
There are many cleaning chemicals available on the market and they differ not only in price and quality, but also in the relation between the price and the quality. It is more and more common for efficient and high-quality products to be available at a reasonable price, which is a very important factor in case of professional cleaning chemicals.
Benefits of professional cleaning chemicals
- high degree of concentration (high content of active ingredients),
- designed for specific application (adjustment of the chemical composition for particular field of use),
- high efficiency,
- operational documentation (manual instructions, technologies, hygiene procedures),
- safety documentation (material safety data sheets),
- low costs of use,
- one packaging of concentrated cleaning agent used instead of several or a few dozens of premixed cleaning agents!
Nowadays, everyone strives to reduce operating costs, including the costs of maintaining the workplace clean. Unfortunately this usually leads to the acquisition of cheaper cleaning products of poor quality which make cleaning inefficient.
The best solution to reduce the costs is to use professional cleaning products and implement adequate cleaning technology based on specialist hygiene procedures.
A dilution formula should be attached to each concentrated cleaning chemical, as both excessive and insufficient amount of cleaning agent will prevent successful cleaning. The application of a correct dosage is facilitated by dosers and dosage systems.
Premixed cleaning products are suitable for the cleaning of small surfaces (20-30 m²) and in facilities where specialist procedures are required – e.g. quick disinfection at the dentist’s or in a beauty salon.
In case of other facilities or frequent use, the use of efficient concentrates is more suitable. It is a perfect solution for those who want to save money.
Apart from the costs, one should take notice of the safety of products. It should be remembered that when working with products which contain sodium hydroxide, it is necessary to wear protective gloves to protect the skin.
Acid products based on citric, ortophosphoric or nitric acids should not be diluted in water of high temperatures. The use of warm water in the solution will increase the rate of evaporation, which may lead to intoxication, especially in poorly ventilated rooms. It is advised to use protective masks if the work involves the use of substances which give off sharp smells or emit vapour.
Another important factor is the product’s environmental impact. VOIGT created its products with environment in mind. The products:
- are easily biodegradable,
- surface active agents meet the biodegradability requirements set forth in the Regulation no. 648/2004 of the European Parliament and the Council on detergents,
- The products introduced into the labor concentrations in the biological sewage treatment plant, cause harmful interference from the operation of the active sediments
- do not contain APEO (toxic phenol derivatives), NTA (complexing agents) and aldehydes.
Cleaning instructions for various surfaces
Granite
Granite demonstrates a relatively high resistance to chemicals and mechanical damage. Its weak point is the polish of polished granite slabs and variable texture and colour of fired surfaces which become matte and lose the original texture and colour as a result of the activity of humidity and sand.
The maintenance of granite is periodic and involves partial polishing, blasting and impregnation which takes quite a lot of time and is expensive, or is performed on a continuous basis by everyday sweeping and cleaning of the surface, periodic washing with a “spray” method and periodic impregnation with polishing. After being swept, the surface has to be cleaned with the use of a two bucket wash kit. You should use cold or moderately warm water (it cannot be hot). Over the summer, you should use slightly basic products and in the winter (if there are salt stains) acidic products.
After you have swept and cleaned the surface, you should clean it using the “spray” method and a small amount of water. You should spray the cleaning agent onto the surface and distribute it using a moist mop. The final step of the process is polishing of the wet surface using a single-disc slow-rotating polishing machine.
Gres
It is very hard and resistant to stains. Despite its low impact resistance it is commonly used as a floor material. In everyday cleaning there is the problem of smudges which occur on the surface and which can be removed using appropriate cleaning technology.
Before cleaning the surface, you should remove loose objects (e.g. sand) from the surface using a vacuum cleaner or a sweeping brush. In case of lime or cement stains you should use acidic products and then carefully rinse and neutralize the surface.
For cleaning on a current basis, you should use slightly basic substances, while for the purposes of thorough cleaning you should use products with higher pH level, preferably slightly alcoholic, to ensure that the floor dries quickly and avoid the need to rinse the surface. For everyday cleaning, the use of a moist mop is recommended. If you use a cotton mop, you should remember that the floor needs to be dried with a dry mop. Polished gres does not absorb water. The excess of water will leave smudges on the dried surface.
Ceramic tiles
There are three types of cleaning ceramic tiles. The first is the initial cleaning which takes place after the tiles are laid. It involves cleaning of the floor and walls to eliminate all remains of glue, grout and lime using an acidic substance based on ortophosphoric or citric acids. You should distribute the cleaning agent on the dirty surface and after 3 – 5 minutes use a mop or a wet vacuum to remove it all. Then you should clean the entire surface with clean water.
The second type involves the current cleaning using the so called moist method and slightly basic substances. The final method is the “special” cleaning recommended in case of neglected floors, which have not been cleaned for a long time or such which contain tiles and joints on which there are hard to remove stains. A cleaning pad should be used in this process and, depending on the type of stains, a strong acidic or basic cleaning agent.
You should remember that ceramic tiles have low mechanical resistance which means you should not use objects with sharp edges for cleaning purposes and each thorough cleaning with the use of highly acidic products should be conducted with good ventilation and be followed by a neutralization (washing with water).
Wood parquet
It is a durable surface, however, its condition also deteriorates with time. This is why apart from cleaning procedures conducted on a current basis, it also needs periodic renovation. The life of the parquet may be extended by appropriate caring methods. These methods include mechanical-chemical treatment (full or surface floor sanding, varnishing, oiling) and chemical-mechanical treatment (careful removal of oil stains and impregnation with adequate polymer-wax products).
In the chemical-mechanical process you should clean the surface manually or using a machine and with the use of slightly basic substances. The aqueous solution of the product should be removed from the surface of the floor as quick as possible. After the stains are removed, you should wait until the surface is completely dry (2-3 hours).
When the floor is dry, you should remove objects which do not cling to its surface by sweeping and distribute a thin layer of the protective product on the surface. The floor should be left to dry for 30-45 minutes.
Carpets
Carpets need to be vacuumed on a current basis and washed from time to time to remove the acarids which can cause allergies. Some rooms of large cubic volume which feature soft floor surfaces need to be vacuumed on a current basis and the carpets need to be washed, preferably using the extraction cleaning method. First, the carpet has to be washed using a vacuum with a special rotating brush. Then, you should spray the carpet and during the washing process rinse the carpet using water with a special substance using a spray-extraction cleaner. The last stage is the removal of stains.
Professional vacuum cleaners for carpets and upholstery facilitate the cleaning process. You can also remove stains by directly applying a stain remover on the stain. It should be done with the use of a cloth by soaking the spot. The cleaning agent should be left on the surface for 2-3 minutes and then removed with a dry cloth until the stain is not visible and the cloth (preferably white) does not get dirty.
The final stage is the neutralization of the stained spot with water. You should carefully rinse the spot to remove the cleaning agent from the surface. If you do not do this, the cleaning agent may cause discoloration on the carpet.
Leather
The procedures which serve to treat leather surfaces include cleaning procedures conducted on a daily basis and specialist procedures, i.e. removing stains and “renovation”. The former involve removing dust from the leather surface, cleaning and polishing (preferably using circular, figure-eight moves).
An example of specialist procedures is stain removal. In this procedure you use a cloth and basic substance which should be distributed on the surface. After a few seconds you should wipe the surface and clean it with water and leave it to dry. It is recommended to check the durability of the leather colorant in an invisible spot before use.
Restoration aims to soften the surface of leather in places where water or a cleaning agent were used with the use of substances based on vegetable oils and antibacterial substances. This substance should be distributed on the surface using circular moves. You can repeat the procedure if the surface is extremely dry. After the substance dries, the surface needs to be polished.
Glass
Glass is a durable material resistant to many, but not all, chemicals. It demonstrates high resistance but can be scratched. The basic cleaning procedure of glass involves a clean cloth (microfiber cloth or other which does not leave dust) soaked in water and a cleaning agent. The glass needs to be wiped dry using a clean, soft cloth or suede or cellulose sponge. The excess of water may be removed using a squeegee. Cleaning is more effective when the surface is in the shade. You should avoid cleaning hot glass or spots which are directly exposed to the sun.
When cleaning glass surfaces you should avoid abrasive or highly alkaline cleaning agents, hard brushes, razors and other objects which could scratch the surface. You should not use petroleum products, i.e. gasoline, fuel oil or lighter liquids. Hydrofluoric and phosphoric acid can cause glass corrosion. You should ensure that the glass cleaners you use are suitable for other elements within the area of the glass, such as sealing agents, aluminum surfaces, wood. Drops or leaks of acids and cleaning agents used to clean metal elements, bricks or walls may damage glass surfaces.
Steel
Elements made of steel are at risk of corrosion (rust) and require routine cleaning and maintenance. Depending on the location of these elements (open space, damp, with high concentration of chemical vapours etc.) and the type of steel there are two kinds of cleaning and maintenance procedures:
Cleaning and maintenance of new machines, not covered with rust: using a soft cloth you should remove dust and other objects. If there are “clinging” stains on the surface, they should be removed with a soft sponge or a cloth soaked with a cleaning agent. When the surface is dry, it is time to perform maintenance procedures. You should soak a clean and dry cloth (or paper towel) with a cleaning agent and carefully wipe the entire surface. In case of clean, non-greasy surfaces, you can proceed to the maintenance process without prior cleaning.
Devices covered with rust: the first step is to remove rust stains. If they are hard to remove, you should use an acidic substance which, diluted or not, should be distributed on the surface. After 2-3 minutes you should use a wire sponge or a scrubber. The surface needs to be rinsed carefully to remove the acidic cleaning agent. The last stage is to distribute the cleaning, care and maintenance agent designed for machines and elements made of steel over the surface (a few times with 10-15 minutes intervals).
Stainless steel
In aggressive environments stainless steel may corrode and this is why appropriate cleaning and maintenance procedures should be applied. For cleaning purposes you should not use substances based on hydrochloric acid, bleaches, silver cleaning products and scrub cleaners. You should not use acids, bases or substances which contain chlorides, bromides and iodides. The use of the above mentioned substances may cause permanent damage to the machines. You should also avoid abrasive materials (e.g. wire sponges) because they may scratch the surface.
Efficient and safe cleaning of steel involves the use of water and soap to remove oil stains, acetate solutions for the removal of limescale, baking soda (coffee sediment) or washing soda (tea sediment), but first and foremost, the use of products intended for chrome and stainless steel cleaning in combination with a soft cloth or a sponge.
Ovens and grills
For the removal of grease stains, especially stains of burnt-on grease, i.e. derivatives of acidic fatty acid, you should use highly basic substances. Depending on the type of stain and the surface to be cleaned there are two types of substances available:
Light, based on glycol ethers - they may be used to clean any hard surface stained with grease, e.g. aluminum, enamel, polytetrafluoroethylene, ceramics and copper.
Strong, containing sodium hydroxide - they should be used for steel unenameled surfaces, as they are quite strong and often contain sodium hydroxide.
Loosened-up stains should be removed with a paper towel. The use of a fabric cloth for the removal of such stains is not recommended due to difficulties with washing.
Acceptable pH levels of the cleaning agent on a given surface
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| Acid area | Neutral area | Alkaline area | |||||||||||||
| Concrete | |||||||||||||||
| Marble | |||||||||||||||
| Granite | |||||||||||||||
| Linoleum | |||||||||||||||
| PVC | |||||||||||||||
| Enamel coated surface | |||||||||||||||
| Stainless steel | |||||||||||||||
| Wool | |||||||||||||||
| Synthetic fibers | |||||||||||||||
Recommended pH levels for the removal of various stains
| Acid environment (0-5 pH) | Basic environment (9-14 pH) |
| Limestone Urine scale Cement residues Water scale Rust | Industrial oils and grease Kitchen oils and grease Other stains hard to remove |
Cleaning sanitary facilities
In sanitary facilities there are a few types of stains you may have to clean. Some of them are:
Grease stains
They occur when the room is used and it is equipped with substances such as soap, shampoos, conditioners, pastes and washing powders. These substances cause grease to deposit on the surfaces, the removal of which requires the use of basic cleaning agent.
If the cleaning agent is a concentrate, its pH level should be between 8-9. The substance is diluted with water (you can use water which is slightly warm) depending on the instructions of the producer. To make cleaning more convenient, you can prepare the substance in a spray bottle. Spray the surface (if necessary, in case of hard to remove stains, leave the substance on the surface for 1-2 minutes) and remove the stain using a sponge or a cloth.
Lime stains
The use of sanitary facilities always involves water, both hot and cold. The water may cause basic limescale to deposit on the surface of sanitary equipment and other elements such as shelves, handles, mirrors. It has to be removed with the use of moderately acidic substances often based on citric acids (pH level of around 3-4 in case of concentrates). First, distribute the substance on the surface and then remove it with a sponge or a cloth.
To remove limescale from a flush toilet in case of everyday cleaning, you can use an undiluted concentrate. It should be distributed and left on the surface for 3-5 minutes. Then using a brush you should clean and afterwards rinse the surface. To brighten the surface of sanitary equipment in case of the presence of hard water, you should use chlorine-based products.
Rust
The entire steel equipment of sanitary facilities (faucets, handles, shelves) requires not only cleaning but also maintenance. Distribute a substance intended for rust removal on a clean surface with a clean cloth or towel and leave it to dry.
If you remove rust stains, the spots which were cleaned have to be polished with a cloth, cleaning pad or towel etc. soaked with an anti-rust substance, which will leave a protective layer on the surface.
Moulds and fungi
Fungi and moulds appear in damp environments where there is a small amount of light, e.g. in bathrooms or sanitary facilities. In these rooms, it is recommended to use acidic cleaning agents intended for the removal of limescale and basic cleaning agents for the removal of grease. If a grey, black or other stain appears on the surface, the first step is to remove the stain using a premixed solution of a cleaning agent which should be left on the surface for a few minutes and then removed with a paper towel. The surface should be rinsed with water.
The next step is to use a disinfectant (a fungicide). The surface on which moulds or fungi occurred should be sprayed with the substance and left for a 5-7 minutes. Then, the surface should be rinsed with water. The procedure should be repeated every two or three weeks and in rooms with poor ventilation more frequently.
Air freshening
The problem of irritating (unpleasant) smells may occur in any used room. It cannot be avoided, but its effects may be reduced or eliminated by deodoration. The process does not consist in masking the smell with the use of common deodorants but in the choice of substances which will absorb and eliminate the nasty smell. Such products are mainly used in:
- sanitary facilities – spraying any surface or spraying the substance inside a room, applying a dosage when flushing the toilet or by means of an automatic doser,
- kitchens – spraying a specialist substance inside sink drains or inside a room, also used as an additive to floor cleaners,
- office facilities – spraying the substance onto the surface which emits an unpleasant smell, e.g. carpet, blinds, also used in car interiors and in travel trailers which are used seasonally.
Disinfection
The aim of disinfection is to destroy disease-carrying microorganisms. Each of us could be a carrier of such microorganisms and any place could be their habitat.
A careful disinfection should involve two stages:
- cleaning the surface, i.e. the removal of organic material,
- disinfection with appropriate substance of adequate concentration.
To maintain a given place hygienic and clean you should:
- take care of personal hygiene - mainly when it comes to hand washing and disinfection according to the instruction. The duration and technique of washing are very important. It is assumed that if washing takes less than 20 seconds, only 40-70% of temporary resident microflora is removed,
- implement appropriate disinfection procedures, especially in sanitary facilities (flush toilets, baths, shower pans, washbasins, faucets and other contact points such as handles, soap and toilet paper containers).
Hand care
The hygiene is ensured not only by cleaning products and chemicals by also by hand care products. The hands are exposed to contact with many kinds of dirt, from dust to grease and oils, and detergents and other chemicals. As a result, they may suffer mechanical damage or become rough, dry and cold. The skin on hands is particularly delicate because underneath there is no body fat. It reduces the skin’s capability of maintaining moisture. Lack of water makes the skin look old, hard, rough and makes it flake and lose all its beauty.
The result of the activity of the above mentioned agents and the changing temperature, dry air, wind or UV radiation is premature ageing of the hands, increased occurrence of corns, skin and nails diseases and general discomfort. The skin of the hands which are exposed to regular contact with harmful external factors requires moisturizing and regeneration.
Washing procedures, i.e. hand washing with warm water and cleaning agents, are equally important. The duration of washing or disinfection should be at least 30 seconds. The majority of people dedicate only a few seconds to these procedures without any knowledge on adequate techniques described in the instruction. To carry out the procedure in a correct manner the use of cleansing agent e.g. soap or gel is necessary.
Skincare soap is available in blocks or as a liquid. It contains a wide range of nutrients and is safe for the skin, which has been confirmed by microbiological and allergy tests.
For those who take special care of their body, we offer antibacterial gel which can be used to wash hands, body and hair. It helps the skin maintain optimum pH level and does not contain soap.
Despite the presence of moisturizing ingredients in hand washing products, the skin loses its natural moisture, especially in contact with chlorinated water. In such cases, we advise to use skin care creams. They revive and moisturize damaged skin and nails. The skin becomes smooth and is protected against harmful agents. They reduce the skin thickenings and improve the way the hands look. Additionally, whilst rubbing the cream in, you can relax.
Foot care
Personal hygiene includes foot care which has a huge influence on their health condition. Many people struggle with excessive sweating of the feet. The sweat may contribute to the penetration of the skin by bacteria and fungi which may cause various foot diseases, e.g. mycosis.
To prevent foot mycosis or eliminate the problem of excessive sweating, you should observe the basic rules of personal hygiene, carefully wipe the spaces between toes after washing and use products reducing foot sweat, such as foot antiperspirants, deodorants or creams. These products reduce the perspiration, eliminate nasty smells, have antibacterial properties and protect against bacteria and mycosis.
Instruction manual for hand washing and disinfection
You should wash your hands following the G.A.J. Ayliffe technique which was approved by the European Committee for Standardization CEN
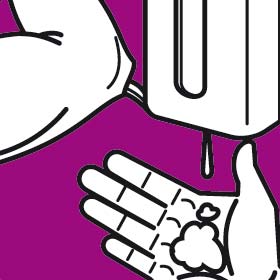

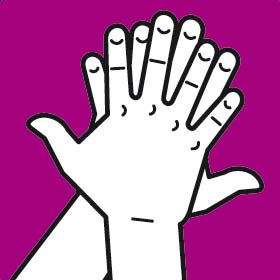
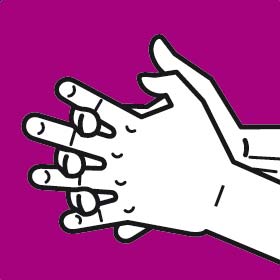

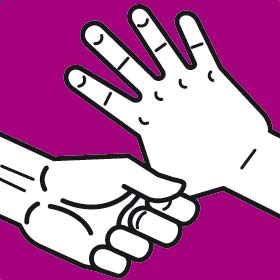
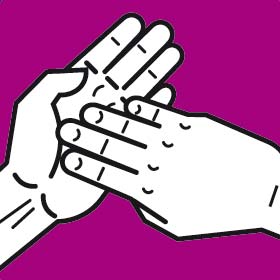
Each step should involve at least five repetitions of the movement. The duration of the whole process should be approximately 30 seconds.
Rinse your hands after washing. Dry with a paper towel or dryer.
This instruction manual was prepared on the basis of PN - EN 1499 standard (hygienic handwash) and PN - EN 1500 (hand disinfection) standard.

Audit Service Trainings
V-TRAININGS
VOIGT was one of the first companies to offer certified practical and theoretical trainings in maintaining facilities in clean conditions. The company approaches each participant individually, and this is why while preparing the training VOIGT takes into consideration all the needs of the client, including the company profile. The training enables one to widen the knowledge on the application of products and cleaning technologies. Through our cooperation with VOIGT, we want to give you the possibility to participate in such trainings.

V-AUDIT
In order to ensure the best selection of cleaning products as well as cleaning technologies for a given facility, VOIGT experts may conduct a thorough cleanliness audit. When the audit is completed, you will receive a detailed technical documentation. By doing so, we give you a chance to reduce the costs of cleaning at your facility.

V-SERVICE
We offer you the possibility to use professional maintenance services provided by qualified VOIGT service men. The experts not only handle the installation and maintenance of dosage systems but also organize periodical visits at our regular customers’ facilities to improve upon the quality and cleaning technologies. The service men use company cars which are equipped with necessary spare parts to be able to promptly remove malfunctions, which enables a smooth intervention after the malfunction is reported.
Contact Advisor!

V-DOSAGE
Reaching the optimum dilution rate of professional cleaning agents is possible only if you use appropriate dosage systems. A correct dosage influences the quality of cleaning work, its efficiency, and consequently the costs and safety of users.
VOIGT offers efficient manual and mechanical dosage systems. VOIGT qualified Service Specialists are always ready to help you with the installation and adjustment of dosage systems and to provide training in the use of the system.
